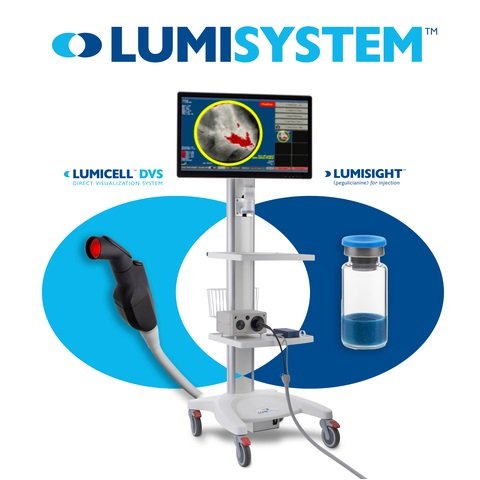
April 18, 2024 – (Newton, MA) – Lumicell, Inc., a company focused on developing innovative fluorescence-guided imaging technology for the detection of cancer tissue during surgery, today announced that the U.S. Food and Drug Administration (FDA) has approved the company New Drug Application (NDA) for LUMISIGHT™ (pegulicianine) optical imaging agent and Premarket Approval (PMA) application for Lumicell™ Direct Visualization System (DVS), collectively known as LumiSystem™.
With a diagnostic accuracy rate of 84%, the LumiSystem enables surgeons to scan the breast cavity in real time after a lumpectomy to detect and remove residual cancer that may have been missed, potentially sparing some patients a second surgery. The LumiSystem combination is indicated for fluorescence imaging in adult breast cancer patients as an adjunct to intraoperative detection of cancerous tissue within the resected cavity after removal of the primary specimen during tumor resection.
The system’s safety was determined based on data from more than 700 breast cancer patients in five clinical studies at top academic and regional community cancer centers in the United States. The most common side effects of LUMISIGHT are allergies and abnormal urine color. LUMISIGHT may cause serious allergic reactions, including anaphylaxis. Findings from the Novel Incisional Surgical Imaging in Tumor Resection (INSITE) pivotal trial to support the system’s efficacy are published in NEJM Evidence.
About Lumicell Corporation
Lumicell is a privately held company dedicated to enabling more complete cancer resection by advancing the development and commercialization of its innovative fluorescence-guided surgical technology. The company’s lead products are LUMISIGHT™ (pegulicianine) and Lumicell™ DVS, which are designed to be used in combination to illuminate cancerous tissue within the breast cavity during initial tumor removal surgery as an adjunct to standard of care. Lumicell’s proprietary pan-tumor optical imaging agent, LUMISIGHT, is also exploring further development for multiple solid tumor indications.













Leave a reply