On April 24, 2024 (Silver Spring, MD & Research Triangle Park, NC), the UTymoKidney™ porcine thymic kidney produced by United Therapeutics was successfully transplanted into a living human body on April 12, 2024, representing several transplant A historic first:
First heterothymic kidney transplant into a living human recipient;
For the first time, a mechanical heart pump was combined with an organ transplant;
First xenotransplantation of living humans using only FDA-approved immunosuppressive drugs.
This transplant is the third xenotransplant using a United Therapeutics xenogeneic organ, following two successful UHeart™ transplants at the University of Maryland School of Medicine in 2022 and 2023.
The transplant received expanded access authorization from the U.S. Food and Drug Administration (FDA), led by surgeons at NYU Langone Health led by Robert Montgomery, MD, PhD conduct. The patient was a 54-year-old woman from New Jersey with heart and kidney failure. A combination of chronic illnesses, coupled with a lack of available human organs for transplant, made her ineligible for a human heart and kidney transplant.
Ms. Pisano is the fourth living patient in the world to receive a pig organ transplant. Two men who received pig heart transplants at the University of Maryland Medical Center in 2022 and 2023 both died within months of receiving their organs. Mr. Richard Slayman was the first patient to receive a pig kidney transplant. He is recovering well and was discharged from the hospital at the beginning of this month.
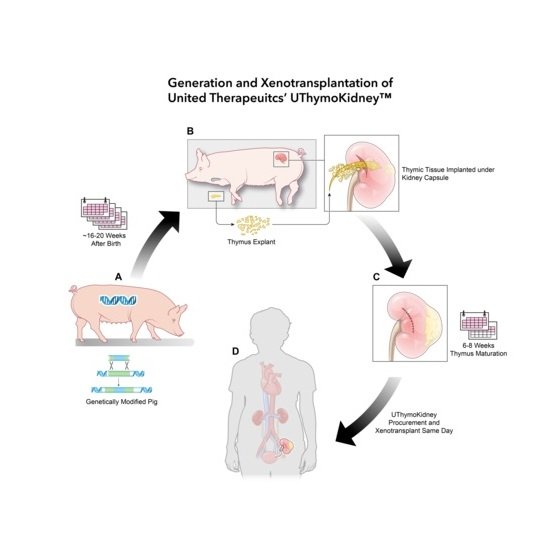
UTymoKidney (single gene-edited pig thymus kidney) This transplant uses a xenogeneic thymic kidney developed by United (the commercial name is scheduled to be UThymoKidney), which contains an under-development xenogeneic pig kidney and thymus tissue from the same pig. The use of pig thymus tissue is intended to modulate the organ recipient’s immune system so that it sees UTymoKidney as “self” tissue, thereby reducing the likelihood of rejection.
The pigs from which UThymoKidney is derived undergo a single gene edit targeting the gene that synthesizes alpha-galactose (α-Gal) on the cell surface, inactivating or “knocking out” the gene. Since alpha-galactose was not detected in pig tissues containing this modification, United called the pig tissue line GalSafe. Alpha-Gal is a cell surface sugar that causes pig organs to be immediately rejected by the body. In December 2020, the GalSafe pig series was approved by the FDA and can be used as a potential source for human food or biomedical use. The transplant surgery announced this time is the first application of the pig series in living patients.
The pig kidney used in the world’s first pig kidney transplant announced last month carried 69 gene edits, while the pig kidney developed by United only carried one gene edit. If proven effective, pigs carrying only a single gene edit could be bred more quickly to quickly address organ supply shortages.
This is United’s third xenotransplantation of a living patient using its xenogeneic organs. To date, the xenograft organs UHearts, UTymoKidneys and porcine kidney UKidneys developed by United have performed 11 xenotransplantations in living and brain-dead human recipients: two patients received UHearts and one patient received UTymoKidney. Six brain-dead patients received UKidney and UTymoKidney, and two brain-dead patients received UHeart.
According to statistics, in the United States alone, more than 100,000 patients are waiting for organ transplants, and 17 patients die every day while waiting for donor organs. The scarcity of transplant organs is a major obstacle that prevents patients from regaining health and is a key problem facing the field. In order to solve this problem, some scientists began to explore cross-species transplant surgery, also known as xenotransplantation.













Leave a reply